
The autumn colours show on the trees, turning a green landscape into shades of yellow, red, orange, and brown. Nights are cooler, with the mercury hovering just above the 0°C (32 °F) mark. But the threat looms when temperatures drop below that point of freezing, which means it’s nitrate season.
That implies concerns with nitrate levels in forages, which is a regular theme to talk about in the coffee shop at this time of year.
Nitrates are primarily a risk for any crop intended to feed livestock. If the crop is intended for grain, it’s not a problem.
While I go into more detail in an article on the livestock half of the nitrate equation I’ve placed in the Keeping & Raising Cows blog, the reason nitrates give cause for attention is the huge risk of death losses.
Nitrates, in high enough levels, can kill quickly. The mode of action is akin to internal suffocation, where the body runs out of oxygen in the bloodstream to the point where it cannot sustain itself, so the animal dies.
Lower doses cause reduced weight gain in growing animals, decreased milk production, and lowered appetite. It can also cause abortion in cows.
This is, no doubt, very serious stuff. It’s as bad as frothy bloat, prussic acid poisoning, ergot poisoning, and fescue toxicity. More on those in the future!
That aside, I would like to introduce to you the forage-half of the nitrate equation.
In this article, I answer frequently asked questions I received when I worked as a forage-beef specialist. These questions largely focused on the timing of nitrate levels in plants and the biggest question of all: When can I harvest?
To answer those questions, it’s essential to first go into the plant physiology and morphology (without trying to bore you to death with too much scientific detail) of why and how plants accumulate nitrates. From there, I discuss the various factors influencing nitrate levels in plants, what plant species are most susceptible to nitrates, and how to test for nitrates. After this, I discuss why and how timing is very important when determining the best opportunity to start greasing up the swather and get out in the field.
What is Nitrate, and How Do Plants Accumulate It?
Nitrate (NO3–) is a highly oxidized, inorganic nitrogenous compound. The chemistry crowd calls it a “polyatomic ion” or anion, but the most important thing is what nitrate does in plants.
Nitrate is the form of nitrogen that plants need for healthy plant nutrition, particularly as they form plant protein and other important physiological functions.
Plants take up most of their nitrogen as nitrate. However, plants cannot use nitrate immediately; first, it needs to be reduced to ammonia (NH3) before anything further can be done, such as forming amino acids.
Once in the plant, nitrate is reduced to nitrite (NO2–) by an enzyme called “nitrate reductase;” this reduction takes place in the cytoplasm of plant cells in the roots or leaves or both, depending on the plant species. The nitrite molecule moves into the chloroplasts of leaf cells (or plastids of root cells), where another enzyme called “nitrite reductase” reduces nitrite to ammonia. Ammonia is quickly used up by the plant cell (since it is toxic to plant tissues if left floating free) to form amino acids and amides for protein synthesis.
Under normal conditions, the nitrate that plants take up in their roots is regularly converted and used for protein synthesis, or, put another way, protein synthesis can keep up with the plant’s accumulation of nitrate. Also, any nitrates that get into the animal’s system are quickly converted by the rumen microbes into ammonia, which is then used as a microbial protein source. (As mentioned above, I will talk more about this in a subsequent post. Also, for more on how ruminants get their protein, see The Wonderful Ruminant Herbivore in the Ruminations Blog.)
It’s a much different story when growing conditions change and thereby affect the regular plant metabolism of N. When conditions shift to where plants become stressed, growth slows (or stops altogether) because the plant is now more interested in sheer survival rather than putting energy into extraneous efforts such as a lot of growth (over reproduction; stressed plants feel a greater need to reproduce as fast as possible before they die to ensure the next future generation continues after the parent plants die). However, the roots just can’t help but keep up taking nitrate.
The problem, therefore, is where protein synthesis occurs, which is usually higher up in the leaves. Photosynthesis and a major portion of protein metabolism occur in the leaves, thus when environmental conditions slow or inhibit these normal plant processes, protein synthesis slows down; yet, root nitrate accumulation does not! The situation becomes akin to a dog chasing its tail or the basic economics of supply exceeding demand, where the conversion into plant protein cannot keep up with soil nitrate uptake.
Plants “need” a shock to their system to slow down or stop normal nitrogen metabolism. These conditions are largely going to be such that slow plant growth:
- Cool temperatures
- Hot temperatures
- Cloudy weather
- Drought
- Frost
- Hail damage
I will expand more on the timing of nitrate accumulation. But as a segue, let’s get more into what conditions–both environmental and plant-related–affect nitrate accumulations in plants.
What Conditions and Factors Affect Nitrate Levels in Plants?
Multiple factors come into play that directly affect the nitrate-accumulation potential of plants. Environmentally speaking, temperature fluctuations, moisture availability, and the availability of sunlight are three of the major influencers; hail damage and frost fall into this category, as well as herbicides. With regards to plant nutrition, high amounts of nitrogen fertilizer–both synthetic inorganic fertilizers and animal manure–that exceed plant needs also play a role. Finally, plant maturity is a compounding factor in plant nitrate levels.
Environmental Factors: Temperature
Optimal growing conditions for plants are between 15 to 25° C (60 to 77° F); this is especially true for cool-season plants (C3 plants). Warm-season plants (C4 plants) can tolerate warmer temperatures, where the optimum is approximately up to 35° C (95°F).
When temperatures rise above these optimum levels, plants begin to feel stressed and focus on survival. The same is true when the mercury dips below plants’ lowest optimal temperature threshold.
Temperatures that dip to 0° C (or lower), especially in autumn, act as a major stressor for plants.
Environmental Factors: Frost Damage
There are some very important variances to know when it comes down to frost. We can divide frost into two main categories:
- Damaging or light frost (temperatures reach -0.5 to -3.5°C (32 to 25.5°F) for a short time before sunrise)
- Killing or hard frost (temperatures reach below -5°C (23°F) for one or more hours before sunrise)
It’s a common myth that a killing frost brings the greatest risk of nitrate accumulation. This is not true. The greatest threat to nitrate accumulation occurs with a damaging or light frost.
Killing frosts kill the plant outright. It acts like a grenade or bomb in plant cells. Remember how water expands when it freezes? Much of a plant’s cell contents are made up of water, which makes rupture inevitable under freezing conditions. Compound that with cell walls, which are quite stiff and inflexible, and you get to a certain point where these walls’ structural support fails, rupturing under the enormous pressure the freezing water or ice exerts. Cell contents are released, and plant tissues die. This is particularly true when the killing frost impacts most of the plant, right down to the major growing points (meristems) of the plant. As a result, the plant cannot bring nitrogen (nitrate) up from the roots as if it were still alive and just damaged by a light frost.
Damaging frosts only cause damage to some plant tissues, mostly at the top of the plant. Damaging frosts also create the same issue as killing frost, where the cells burst due to the freezing water expanding as it turns into ice, but only to some parts of the plant. It doesn’t stop growth; just temporarily impairs it. Tissues can still function, even though the stress damaging frost has put on the plant shifts its metabolism into survival mode. As a result, nitrates can accumulate in the cells and move into the still-functioning, albeit injured, leaves.
I would like to share with you a very good tip to memorize when you’re out in the field checking your crops before harvesting them for forage (note: this only applies to cereals such as oats or barley that are headed out and at the milk to the mid-dough stage). Take a kernel and squeeze it between your thumb and forefinger until you get liquid coming out. If it comes out milky, the plant is still alive and can still accumulate nitrates. If the liquid comes out clear, the plant is dead and, therefore, safe to harvest. The clear liquid indicates that the plant is no longer continuing to put its resources into further maturity of the plant because it has died due to cell rupture.
What about the lower leaves that don’t get damaged by frost? They are also stressed, but it’s not because of freezing temperatures; it’s much more to do with light or lack thereof. The leaves shaded below the frosted upper portions also accumulate nitrate, making the potential for nitrate reduction in the lower half of the plant rather limited.
Environmental Conditions: Sunlight Availability
Cloud cover, shading, or simply just surviving through the night will cause nitrate accumulation in plants. All of these are stressors on the plant because they slow down photosynthesis.
We don’t notice this because of the timing (see below) of accumulated nitrates reaching peak levels and since many of these conditions are short-lived. For example, nitrates quickly dissipate after the sun rises, and the day remains bright and sunny with optimum growing temperatures. A couple of cloudy days where nitrates will accumulate is countered when they move off or evaporate into nothingness, exposing the sun to the slightly light-starved plants below.
It’s a much different story when these cloudy days extend for a week or more.
Smoke from forest fires that obscure the sun’s rays for a long period slows photosynthesis, just as cloud cover does.
Yet, ironically enough, plants need that cloud cover to bring them the moisture they need in the form of rain. These poor plants are sort of at a crossroads like Marie Antoinette: you can’t have your cake and eat it, too! (Except if some lucky plants get irrigated on a sunny day.)
Environmental Conditions: Moisture or Lack Thereof
A lack of sufficient soil moisture can cause nitrate accumulation in plants. Since moisture is needed to transport nutrients up into the plant, maintain plant cell rigidity, and perform other important physiological processes such as protein synthesis, the lack of moisture inhibits this ability, causing plants to feel stress, thus creating a nitrate-accumulation issue.
The key element here is not the lack of rainfall for an extended period that creates the nitrate-accumulation issue but rather the soil’s water-holding capacity, which contributes to an effective water cycle. There must be a notable loss in soil moisture that causes plants to feel water-stressed and, therefore, accumulate nitrates.
While this is an entirely different topic that I don’t want to delve too deep into, particular factors that create this include a lack of dead and living plant residue armouring the soil surface plus insufficient soil organic matter to capture moisture and soil structure. Compacted, block-like or plate-like structures instead of porous and column-like ones where water can trickle down deep into the soil profile are troubling.
All you need to do is pay attention to where the water goes in a big rain event. There’s a big effective water cycle problem (folks in Holistic Management call it a “noneffective water cycle” as opposed to an “effective water cycle”) when much of the water runs off into adjacent water bodies (ephemeral or permanent ponds, sloughs, dugouts, creeks, rivers, lakes, etc.) instead of quickly soaking into the soil where it should remain for a long time.
Drought is a situation in which plants experience a double-whammy of environmental stressors: hot weather and lack of soil moisture (the latter caused by insufficient plant residue that cannot protect the soil, thereby encouraging greater evaporation and heating of the soil surface, which quickly dries out the soil). Plants stop growing because conditions are unfavourable for growth.
We already understand that stressed plants who stall growth will accumulate nitrates, however, there is some highly cautionary empirical evidence out there that shows that plants–especially those that are immature–pose a very dangerous risk of nitrate accumulation when they receive rainfall after a lengthy period of drought. However, it’s wise to note that much of such evidence was noted in warmer climates, such as in India, New Zealand, Australia, and the southern United States. Little, if any, applies to more northern areas that have shorter growing seasons, such as here in Canada.
While rains spell a marked reprieve from drought, hail is a whole different matter and certainly not the type of reprieve anybody would be looking forward to!
Environmental Conditions: Hail Damage
Just to avoid complicating things with regard to hail, let’s assume that the weather has been highly favourable for growing forages (as opposed to a drought situation discussed above)—until a wicked hail storm arrives, that is!
Hail damage acts much in the same way that frost does: it reduces the capacity of the plant to photosynthesize. The bottom half of the plant (roots) are unaffected and continue pumping nitrates into the plant as normal. However, the upper part of the plant cannot use the nitrate as it normally can before hail (or frost) because of the damage it caused to the very important green photosynthetic solar panels. How much this capacity is diminished depends on how significant the hail damage is.
Light hail damage, where some of the leaves were stripped but plants weren’t completely annihilated, means that there will most certainly be a period of time when nitrates accumulate as the plants heal themselves and regrow after the weather-related injury.
If there’s a total crop loss, however, nitrates will still accumulate in plants that eventually die due to a completely eliminated ability to continue photosynthesis. Nitrates will remain in this plant material for a very long time.
Frost, as Mother Nature’s all-natural organic herbicide, and The Great White Combine (hail), all act in the same way as a more man-made chemical intended to selectively kill certain plants: herbicide.
Environmental Conditions: Herbicides
Herbicides can be a nitrate-accumulation nightmare. However, this largely depends on the chemical mode of action. Not all herbicides are created equal; there are well over a dozen (12) separate classified groups of herbicides, separated by their different modes of action.
With regard to nitrates, most attention must be paid to the groups that specifically target a plant’s growing points and those that affect its photosynthetic capacity yet do little to affect the roots. This is because the non-systemic (foliar) mode of action actively kills the top growth rather than systemic herbicides that translocate down into the roots and interrupt the plant’s system from the bottom up.
Top-growth-killing herbicides interrupt the photosynthetic activity and do it quite quickly. Some may move down into the roots, but usually not enough to affect them. This leaves the roots (pun intended) able to continue normal processes of collecting nitrates to send up into the rest of the plant, but just like with the impacts of hail and frost, the herbicide has damaged the upper portion of the plant so much that it can’t use the nitrate for protein synthesis. Thus, nitrates accumulate.
The most notable herbicides that will do this are 2,4-D, MCPA, Basagran, Pardner, Vanquish, Grazon, Tordon 22K, Curtail M, Lontrel, and potentially (though less likely than the aforementioned herbicides), Restore II and Reclaim II.
Reglone is another herbicide (used as a desiccant rather than a means to target weeds) that carries a significant risk of encouraging nitrate accumulation in forages. Reglone is used to terminate plant growth by quickly killing the top growth; it acts very much like frost or hail does, except it’s a man-made chemical.
In case someone asks, “What about glyphosate or RoundUp?” After talking with a couple of herbicide crop specialists, the general consensus of whether this herbicide also creates issues with nitrate accumulation is that no, it does not. Glyphosate basically acts systemically, where it targets the whole plant, starting at the roots and working its way upward. When it does this, it basically slowly shuts down the physiological processes from the roots upwards, inhibiting the plant’s ability to take up excessive nitrates. Thus, when the plant finally dies, it dies due to the slow progression of the effects of the herbicide and results in zero locked-up nitrates.
However, caution needs to be exercised when choosing what herbicide to use on which plants. If you have any detailed questions about herbicidal use, it’s best to talk with a local agricultural extension person for more information. Also, always read the label before using it. Certain restrictions must be adhered to when it comes to knowing when it’s safe to graze or feed the treated crop.
With all of these environmental variations, one has to wonder if all plants are inclined to accumulate nitrates, or are only certain plants more predisposed to be nitrate accumulators than others? Let’s talk about that next.
Botanical Conditions: Plant Species
All plants take up nitrate at some level (or ammonia) to be converted into plant protein. However, certain plants are much more likely to readily accumulate nitrates than others.
Here is a list of the most likely culprits to keep your eye on:
| Annual cool-season crops: | Annual warm-season large/small-seed crops: | All Brassica species, including: | Most introduced perennial cool-season grasses, including: | Various weed species: |
| Barley | Corn (Maize) | Canola/Rapeseed | Timothy | Pigweed (i.e. Redroot) |
| Wheat | Sorghum | Forage Rape | Orchardgrass | Dock (i.e. Western Curly) |
| Oats | Sudangrass | Mustards | Brome-grasses (Smooth, Meadow, etc.) | Kochia |
| Rye | Sorghum-sudangrass | Turnips | Fescues (Tall, Meadow, Creeping Red, etc.) | Lambs-quarters |
| Triticale | Millets (all varieties) | Collards | Wheatgrasses (Tall, Intermediate, Slender, Quackgrass, etc.) | Thistles (Canada, Russian, Bull, etc.) |
| Spelt | Radishes | Reed Canarygrass | Nightshades | |
| Flax | Sugarbeets (tops) | Foxtails (green, yellow) | Smartweed |
- Other weed species:
- Jimsonweed
- Fireweed and other willowherbs
- Johnsongrass
- Wild sunflower species
- Barnyard Grass
- Mustards
- Ragweed species
A common question is whether legumes, such as alfalfa, clovers, peas, and beans, are apt to accumulate nitrates. They are, but only if they are given a very high amount of fertilizer. Otherwise, in normal situations (including those where they are given adequate fertility via manure or synthetic inputs), they are not a risk at all. The primary reason is that they have root nodules that are created by their partnership with Rhizobium bacteria that assist in regulating the amount of nitrate that goes up into the plant. Instead of an unregulated amount of nitrate being pooled up in the plant’s leaves and stems, the root nodules act as the nitrate pools instead, allowing only enough at a time according to the rest of the plant’s needs. This makes nitrate accumulation in legumes a rare occurrence.
It’s worth noting that not all species will accumulate nitrates at the same rate or carry the same risk of nitrates as others. For instance, many sources state that weeds like those listed above are at a much greater risk for containing nitrates than other domesticated annual or perennial species. Corn and sorghum have been noted in most publications from the United States as having a lower risk of nitrates than cereal crops. Finally, some sources stated that many perennial cool-season grasses pose little to no risk over annual cereal crop species.
These claims–as I will call them–often forget two very important factors that I will address below: the first is the growth stage, and the second is the application of nitrogenous fertilizers. The latter carries the greatest influence on nitrate levels in plants and can mean the difference between not being concerned at all and risking great economic losses if the feed is not tested before feeding.
Botanical Conditions: Stage of Growth
Immature plants pose the greatest threat to nitrate toxicity than mature plants. This is because these plants are focusing a lot of energy and protein synthesis on developing more leaves, as well as what will eventually become flowers (or inflorescences) and seeds. Plenty of protein synthesis activity will be happening in the leaves, especially the newer flag leaves at the top of the vegetative plant. Grasses grow by pushing the youngest leaf to the top from the base and the very centre of the plant, thus a lot of nutrients, including nitrate, is pulsating up into this new shoot so that it will grow to maturity. The final seedhead pushes up the same way and heralds that a plant is nearly at the end of its life.
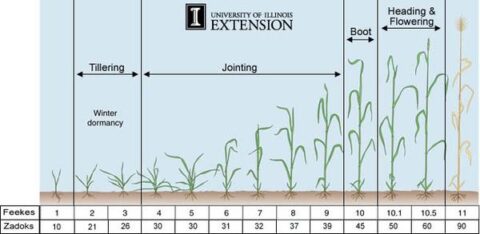
Vegetative plants or tillers from older plants that range from the two-leaf stages to the boot stage are a particular risk; plants that are pre- and post-flowering and starting to fill out their seeds are also a risk, but less so than when they were still in their vegetative stages.
These young plants are particularly susceptible to accumulating nitrates when they return from a long dry period. This is a big problem in areas with longer growing seasons, such as in Australia and India, because the new growth is taking advantage of a sudden bout of moisture, plus the fertility available, largely as some sort of nitrogen source. The rains after a drought for these parts of the world give producers a false sense of security, and Sidhu et. al. (2011) discussed in their paper (see Sources below), there were significant death losses as a result of high nitrate concentrations in the young, seemingly-grazable plants.
In colder climates (such as here in Alberta, Canada), with more defined seasons (as opposed to wet versus dry), this is perhaps less of an issue unless there is a sudden rush of growth after a particularly dry spell during the spring into summer, and where fields were fertilized last fall or possibly that spring. However, for this to happen, the young plants will have to have felt some level of stress for this to happen, such as a late frost or a dry period right when they are young and growing. While I shouldn’t say that this will never happen (because it certainly can happen), a scenario such as this is virtually so rare that it’s not something to be worried about.
However, it’s still a precaution to take when young plants are coming out of a very dry summer and responding to late-season rains. There is still enough sunlight and warmth during the days for these plants to grow during the autumn right up until a killing frost, making these young plants still a risk for nitrate accumulation and thus poisoning livestock.
What about more mature plants? Because they’re nearing the end of their life cycle, they’re not so intent on pushing up nitrates to the top as with younger plants. Instead, much of the nitrate, if they have not reached full maturity, will be more heavily concentrated at the bottom third of the plant. The top third, going into the grains, will have the least amount of nitrate concentration. Plants that have reached full maturity (hard-dough stage) have virtually no nitrate in the seeds whatsoever.
Managerial Conditions: Plant Access to Fertility
Synthetic nitrogen fertilizers and manure are perhaps the most influential of all other factors when it comes to nitrate accumulation and nitrate toxicity. Whether or not the pasture or field was fertilized–and how much was applied–will depend on how long you will need to wait before you can harvest for forage or graze.
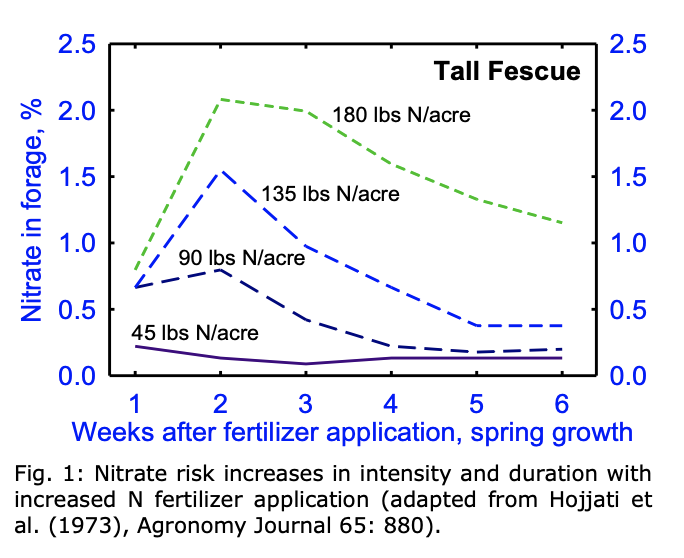
As a general rule of thumb, the more fertility that was applied to the field, the greater the chance of nitrate toxicity will be present. Fifty pounds (22.7 kg) or less of actual N is a rate I would be comfortable in stating that nitrate risk is low; between 50 to 90 lb (22.7 to 40.8 kg) per acre of actual N is still less of a concern than a rate of 100 lb (45 kg) or more of actual N (NOT the actual product or the actual amount of fertilizer applied to the field; see Manitoba Agriculture’s Soil Fertility Guide for help with fertilizer calculations.)
Most fertilizer applications are done in the spring versus later in the year, like in summer or fall. This usually indicates to me that, especially with rates that are less than 100 lb (45 kg) of actual N, the plants will have used up most of the nitrogen from spring applications versus those later in the year, thus carry less of a risk for nitrate accumulation by the time autumn comes around. However, you cannot be absolutely sure until you get them tested for nitrates.
In most farming operations, most pastures and haylands almost never get fertilized, and if they do, in my line of work, I have not heard of fertilizer applications being all that significantly high; certainly not to the amount that is commonly applied in growing corn, wheat, canola, and other crops intended for the grain market. (Regarding growing corn here in Alberta, most of the fertilizer cost is for growing corn to graze or chop into silage; conditions are just not suitable for grain production in this part of the world.) Thus, nitrate risk is low for such areas. Again, the exception is if a heavy amount of fertilizer or a large amount of manure was also applied.
Manure is a whole different beast in itself. A large portion of manure is organic matter, and not much of it (when you compare it with concentrated pelleted fertilizers such as urea [46-0-0]) is an actual fertility product; where urea has 46% actual N, the average percentage of N for manure from beef cattle is only around 1 to 2%.
It would have to take a LOT of manure (say in excess of 9,000 pounds of actual product per acre!!) to provide the same amount of recommended nitrogen to the stand compared with the amount required to apply 46-0-0 fertilizer. However, this is still different if the land was grazed (no manure was spread from the home corrals; it was all dumped there by the cattle that were grazing away) and in such a way where the manure was, as much as possible via temporary fencing, spread evenly across the land with a large concentration of animals being grazed per unit area. When this is the case, that means a lot of nutrients available for plants to uptake at will… which means potential for nitrate accumulation.
How much the manure or fertilizer has contributed to the uptick in nitrates in the forages is a question best left for the next section, which will include some tips on testing your forages for nitrates.
How Do You Test for Nitrates in a Forage Stand?
There are two primary methods you can use to test for nitrates in a forage stand. The first (and easiest) is collecting plant samples to send away to a lab. The second is using a nitrate testing kit that you may be able to get from your local county office or neighbour. For the purpose of this post, I won’t go over the second method, as the kits available vary from wherever you live; also, some counties may not have kits available at all, and you may have to resort to sending forage samples away instead. For that reason, I wish to expound on the first testing method, as it’s important to know how to get a satisfactory (or at least accurate) test result back after sending some forage samples away.
Sending a forage sample away to a lab is the most recommended because not only do you get nitrate test results back, but you also get your forage analyzed for nutrients as well–protein, energy, and macro-minerals especially (most recommended). It’s also ideal if you plan on grazing the stand that you have tested in a week or so. The only drawback with sending a forage sample away to a lab is the time that it may take to get a result back; the lab may be experiencing a back-log in forage samples from other parts of the country, and test results may not come back until several days to a few weeks later; this can be problematic if you’re wanting to start grazing very soon, and cannot wait much longer for the lab to get back to you.
If you’re taking plant samples from a swath–ideal, but only if made the last couple of days–take at most five to six (5 to 6) different plants from various parts of the field, not just one spot. Take one or two from up on a hill, another couple down in a valley area, and others mid-way on an incline, and so on. This is so that you get a representative sample from around the field to send to the lab.
If you’re taking samples from standing plants, cut the plants 4 to 6 inches (10 to 15 cm) from the soil surface, also one or two in various locations around the field: on top of the hill, in the bottoms, mid-slope, and everything in between. You only need at most 5 or 6 plants to send off. Do not take them by the roots, as this will skew the nitrate values the lab technicians get when they’re analyzing your sample to much higher levels than what would be noted as average!
With standing corn, all you need is about a half-dozen plants taken similar as described above (cut ~6 to 8 inches from the soil surface) and a wood chipper. Put the plants through the chipper, then collect two-thirds of a bread bag full to send away. A forage harvester works just as well, just as long as there’s a way to effectively collect the chopped corn plants and then gather a sample from that pile.
When it is crucial, I highly recommend taking fresh forage samples from the field three to four days after the hail or frost damage has occurred. This way, you can get an accurate reading on how high the nitrates have actually gotten in your feed and then figure out how to introduce your animals to this crop from there.
What About Taking Samples from Stored Feed, like Hay Bales or a Silage Pile?
Taking forage samples from stored feed is unlike taking sample plants from an existing stand. For both bales and silage piles, you will need a forage probe coring tool to collect your samples from. A hand-grab sample will not give an accurate analysis of what you need, as it tends to be from a part of the bale, pit, or pile that is the most weathered and subject to external forces that will change the forage quality.
You will need the short ~3-foot long (0.75 m) probe for coring large round or large square bales hay bales (smaller for small square bales) and a long probe (~6 feet [2 metres]) for a silage pile. Unless the forage coring kit you borrow from the county, neighbour, or an applied agricultural research group has a manual turn-crank, it’s best to attach it to a power drill to get into the bale or silage pit.
With the bales, position the probe in the middle of the bale between the strings–not at the non-stringed ends. Then push in until you get to the centre of the bale. When you pull out, you should get a nice core sample. Repeat for about 10 bales out of 100 bales (or, for instance, if you have 250 bales, collect 25 randomly selected cores), putting each core in a 5 gal. bucket as you go. Each bale should be randomly chosen, not one after another in a single row or a certain part of the field (if the bales have not been trucked in from the field yet). Once you’re done, mix up the cores well, then take half to two-thirds of a bread bag full of the collection to send away to a lab for analysis.
With a silage pile or pit, it’s more of a matter of finding 10 random spots to take a core from near the middle of the pit and patching up each spot with silage tarp tape (important if you’re coming through the silage plastic, versus doing so on a freshly-made and packed pile). Then, just like with the collections obtained from the bales, mix them up and take a bit over half (not full) of a bread bag full of silage material (make sure you pick out the plastic bits, though) to send off to the lab.
How do you Keep Samples Fresh so They Don’t Deteriorate During Shipment?
It’s important to ship them off as soon as possible, as in the next business day of taking the sample. DO NOT ship samples late in the week (as in on a Thursday or Friday), as they may be delayed in getting to the lab in time.
A few tips to keep samples safe and secure if they have to be held or shipped.
- Keep them cold by freezing them for 24 hours before sending them off. This is highly recommended for silage or fresh samples. Keep them in air-tight plastic bags. Send them off in an insulated container to keep them frozen until they reach the lab.
- Dry samples (as an alternative to freezing) by laying them out in a thin layer on paper or paper towels. Artificial heat (such as drying them in an oven) is also desirable, but don’t allow the heat to get greater than 70°C (160°F) lest they begin to burn, and you have nothing but cinders left to send away. Silage, however, should be kept frozen, and not be dried. Drying is recommended with fresh forage, hay or straw. Fresh forage will ferment if left in an air-tight bag without being frozen or dried immediately after collecting samples.
Failing to do any of these practices risks samples losing their nutrient value (or spoiling in some way, shape, or form) and results in nitrate losses, skewing the results. It makes the lab techs happier. They have good samples to deal with, and you are happier because you don’t have to go out and resample again when you could be spending your time doing better things.
You can find a forage testing lab that’s local to you to send samples to and give them a call to see what they charge for testing nitrates and other nutrient analyses. Or, you can ask your county office or local fertilizer dealer to send the samples away for you and deal with the paperwork and hassle of sending them off via the right mail or package carrier. It’s all up to you. If you were to ask me what I recommend, I wouldn’t have a recommendation for you. (Although personally, if it were my farm, I’d take the time to find a good reputable forage lab to ship to and go from there… especially if I want to know what the protein, energy, macro- and micro-mineral contents of my forage samples are in addition to nitrates… that’s just me!)
So, now that you’ve understood all the factors that go into how plants could accumulate nitrates and are well informed about how to take samples, it’s now time (… get it?) to figure out the timing aspect of nitrate accumulation and risk for toxicity to livestock. It’s about time we get into that! (Alright, I’ll stop with the untimely lame pun jokes…)
Timing Important to Harvest or Graze Potentially Nitrate-Bearing Forages
This is the biggest conundrum of all: When can I harvest?? When can I graze??
The time frame for nitrates is fairly easy to figure out when you understand this:
Nitrate levels reach their peak 5 to 7 days after a hail or frost. They settle back down to normal levels 5 to 7 days later.
Specifically, with hail or frost, that means you have two to three (2 to 3) days post-hail or post-frost event to harvest the crop, to get that swather gassed up, greased up and out in the field cutting down that crop, or sit and wait for 10 to 14 days until the nitrate levels are for sure back down to normal.
If you’re grazing cattle, it would be best to wait for those 10 to 14 days out, just to be safe, or until the test results return and give you the green (or red) light. I will talk more about this in the next segment, including how to read the nitrate test results you will have received.
I’m not sure if the science is still well understood about nitrates, but the reason I suspect that it takes this long for nitrate levels to reach their peak is that it takes a bit of time for nitrate accumulation levels to reach peak while the roots continue to supply the leaves and stem yet cannot keep up with this supply due to the damage it has to first deal with. The best way I can explain it is by using the factory analogy.
Think of the above-ground portion of the plant as a factory, where goods are shipped in, created to make different things (such as amino acids), and then shipped out. The roots are a storage warehouse that sometimes has a communication issue with the factory. In this case, the warehouse didn’t get the note that the factory was damaged, so it kept shipping goods (nitrates) like normal to the factory.
The factory storage area is doing okay for the first couple of days, but the storage area gets noticeably more and more full as the days go on; not only that, but the damaged factory’s production line can’t keep up with the warehouse’s constant supply! Thankfully, builders are working as hard as ever to get the damaged factory fixed so that by the time the day comes when the factory’s storage area just can’t hold those packages any longer, production has just started picking up again and gets in gear to catch back up to the warehouse’s supply line.
Frost is perhaps one of the more interesting nitrate scenarios I’ve had to do mental gymnastics on. It’s not always guaranteed that the next frost (if an area gets a damaging frost) will come at the right time, such as two weeks after the first damaging frost, especially if it’s a killing frost. Subsequent damaging frosts make life much more interesting compared with a damaging frost than a killing frost. Consecutive damaging frosts may prolong the nitrate timeline, depending on when they arrive. But when it comes down to killing frosts, you either get the luck of the draw, or you don’t.
A killing frost will be much more unsettling if it occurs 5 to 7 days after a damaging frost. This is because that killing frost is killing a plant when nitrate levels are highest, locking those nitrates in, and there’s nothing you can do about it. However, if it arrives 10 to 14 days after the damaging frost, you’re in the clear because it has killed the plants at a time when nitrate levels are low and killed the plant so that nitrates are unable to accumulate up into the leaf/stem tissues.
Drought is a bit easier to figure out. During a drought, nitrates have already been building up over time but spike significantly when the rains arrive—particularly true with young plants, which happens over a matter of a few days. About a week later, with better-growing conditions than before, nitrate levels will have settled back down to be deemed safe for grazing or harvesting.
Conclusions
Overall, timing and being able to answer the question, “When is it safe to harvest or graze?” really depend on your context. I suggest that if you still have questions, contact a local agricultural extension agent or shoot me a private inquiry.
In the next part of this two-part nitrate series, I talk about the animal side of the nitrate equation, from the science behind how nitrates affect animals to some feeding guidelines and how to read those nitrate test results.
I wish you the best this harvest season!
Sources
Alberta Agriculture & Forestry. Crop Protection 2020. https://www.alberta.ca/crop-protection-manual.aspx
Alberta Agriculture & Forestry. Nitrate Poisoning and Feeding Nitrate Feeds to Livestock. https://open.alberta.ca/dataset/64f872db-21d1-4545-a2ea-f6420ff7b5be/resource/e702330a-a122-4edb-bf5d-1d6c947e0a16/download/1991-400-60-1.pdf
Cornell University Publication Series. Drought and Risk of Nitrate Toxicity in Forages. http://nmsp.cals.cornell.edu/publications/factsheets/factsheet70.pdf
Manitoba Agriculture. Nitrate Poisoning. https://www.gov.mb.ca/agriculture/livestock/production/beef/print,nitrate-poisoning.html
Nebraska Extension. Nitrates in Livestock Feeding. https://animalscience.unl.edu/Our_People/g1779.pdf
Saskatchewan Agriculture. Nitrate Toxicity | Animal Health. https://www.saskatchewan.ca/business/agriculture-natural-resources-and-industry/agribusiness-farmers-and-ranchers/livestock/animal-health-and-welfare/nitrate-toxicity
Sidhu, P.K., G. K. Bedi, Meenakshi, V. Mahajan, S. Sharma, K. S. Sandhu, and M. P. Gupta. 2011. Evaluation of Factors Contributing to Excessive Nitrate Accumulation in Fodder Crops Leading to Ill-Health in Dairy Animals. Toxicology International. 18(1): 22-26. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3052579/#:~:text=Nitrate%20accumulation%20varies%20with%20the,nitrate%20than%20the%20mature%20crop.
Undersander, D., D. Combs, R. Shaver, D. Schaefer, and D. Thomas. 2020. Nitrate Poisoning in Cattle, Sheep, and Goats. University of Wisconsin-Madison Extension. https://fyi.extension.wisc.edu/forage/nitrate-poisoning-in-cattle-sheep-and-goats/
University of Minnesota Extension. Plants that accumulate nitrate: a potential problem for horses. https://extension.umn.edu/horse-pastures-and-facilities/plants-accumulate-nitrate-potential-problem-horses
0 Comments
Trackbacks/Pingbacks